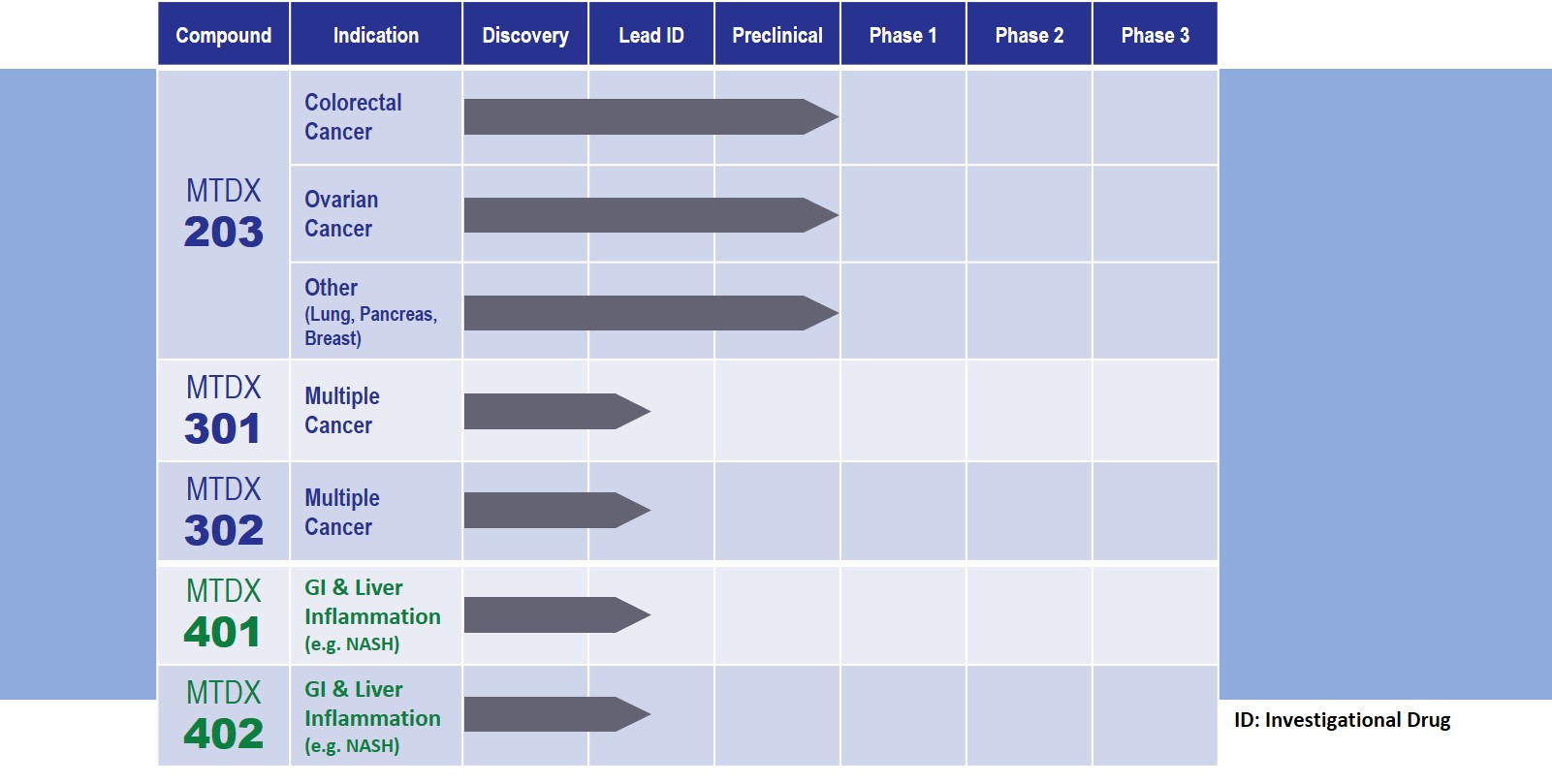
MTDX 203: Our lead compound targets colorectal and ovarian cancers
In a clinical Phase I/II study of pentamidine + standard therapy in advanced colorectal cancer that failed at least first line of therapy, a subset analysis of median overall survival response in relation to the EE level indicated that pentamidine was more effective in cancer that expressed a high level of EE.
Moreover, our subsequent studies in colorectal and ovarian tumor models have shown MTDX 203 to be a better EE enzyme inhibitor and a better anti-cancer agent than pentamidine, when used alone or in combination with Standard of Care agents or PARP inhibitors. MTDX 203 has shown to be at least 3.5x more potent as an EE inhibitor and resulted in 20x more cytotoxic activity on these cancer tumors.
These findings potentially make MTDX 203 first-in-class among the compounds directly acting on the DNA damage repair pathway. We are currently working on an Investigational New Drug (IND) submission in Canada and in the United States to move MTDX 203 forward into a Phase 1 clinical stage.
Other anti-cancer compounds
Montdorex also has a library of follow-up anti-cancer compounds, including MTDX 301 and MTDX 302, currently at early stage of development.
Other anti-inflammation compounds
We have also taken the dual function nature of Pentamidine as an EE inhibitor and anti-inflammatory/Lipopolysaccharide (LPS) binding to broaden our scope of indications into GI and liver disease. MTDX 401 and MTDX 402 are currently in drug discovery phase. The effect of pentamidine as potential anti-inflammatory agent is well documented. However, its development for anti-inflammation application is limited by its cytotoxic effect.
With proprietary technology, we are able to identify pentamidine analogs that retains the anti-inflammation activity but reduce cytotoxicity to broaden our scope into gastrointestinal (GI) condition as well as liver disease.


